- Home
- Why Truffles?
- Blog
- You Can't Run from Vein Disease
- VenaSeal
- Summer is Almost Over
- IDK about Vein Disease
- Vein Treatment Fayetteville
- Sonography to RN Degree Program
- Vein Testing Differences
- Advantage of Vein Treatment Before Knee Surgery
- Poor Medical Advice
- Green Tomatoes for Varicose Veins
- VenaSeal Treatment Atlanta
- Fake News
- Iliac Vein Compression
- Vein Treatment
- DVT
- The Vein Specialists
- Vein Treatments
- What is Vein Disease
- Vein Conditions
- Vascular Testing
- Look and Feel Your Best!
- Reviews
- Media
- Contact Us
- Schedule and Appointment
- Free Vein Screening
- Give Us Feedback
- Referral Resources
- Make a Payment
Experienced VenaSeal Providers in Atlanta

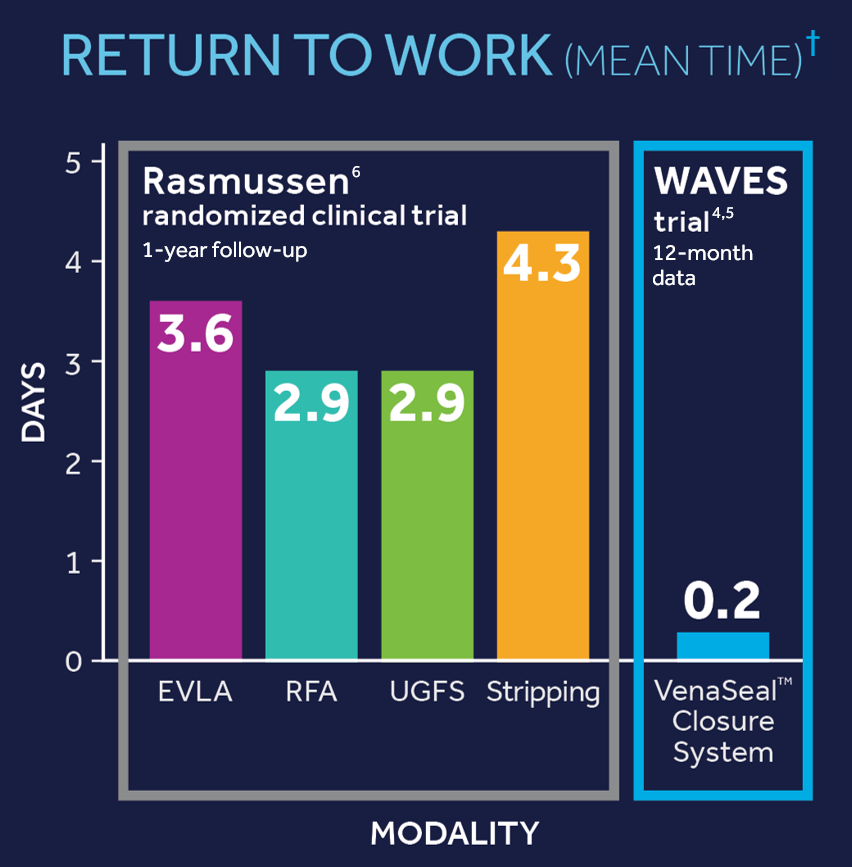
VenaSeal™ Closure System
Cyanoacrylate Adhesive to close the diseased vein

As a new procedure, the Venaseal™ by Medtronic does have coverage by Medicare and some private insurance companies. The procedure is FDA approved and has shown great results in 4 clinical trials. Due to the procedure being new patients who want it must pay out of pocket.
The glue or chemical adhesive that Venaseal uses is a proprietary adhesive that has been used in surgical procedures for decades. The advantages of Venaseal™ is that there is no anesthesia! You are not required to wear compression before or after the procedure.
Once you have a detailed venous duplex exam Dr. Feldman will talk to you about the results and develop a treatment plan specific to your needs.
Venaseal is a great option for those who don't want to wait, have a high deductible or want to look and feel better immediately. The procedure consists of one needle stick and since there is no thermal ablation can be performed along the entire length of the vein. Patients literally can tell a difference when they walk out the door.
Not every patient is suitable for the Venaseal™ treatment. As such all patients will have to undergo our usual consultation and a venous duplex ultrasonography to find out exactly what is going on inside the legs and find out which veins are involved before a decision as to whether Venaseal™ treatment is the right choice in their legs.
The Venaseal™ treatment by Medtronic is a new way of closing the main vein in the leg that causes varicose veins (usually either the great saphenous vein GSV or the small saphenous vein SSV or both). When the Venaseal™ or “superglue” treatment is performed, only one needle hole is needed per vein treated as there is no need for the additional injections of local anesthetic around the vein – unlike endovenous laser ablation or radiofrequency ablation.
However, although the early findings do look promising, the Venaseal™ or “superglue” treatment for varicose veins has not been around long enough to have shown that it keeps the vein is closed in the medium to long term. Clinical results show great results 18 month after clinical trials and the numbers are actually better than other treatments in some of the studies. Truffles Vein Specialists was the first practice in the state of Georgia to perform the VenaSeal procedure.
Do you have vein disease? Find out now!
Medtronic Press Release
New, Innovative Technology Brings Relief to Patients Suffering with Varicose Veins
DUBLIN - November 9, 2015 - Medtronic plc (NYSE: MDT) today announced U.S. availability of the VenaSeal(TM) closure system, the first and only non-tumescent, non-thermal, non-sclerosant procedure approved for the treatment of symptomatic venous reflux in the U.S. The VenaSeal closure system was approved through the FDA's Pre-Market Approval (PMA) process, and is a minimally invasive procedure that uses a proprietary medical adhesive to close superficial veins of the lower extremities, such as the great saphenous vein, in patients with symptomatic venous reflux.
"Patients are often told their varicose veins are only a cosmetic issue, but varicose veins are a sign of a condition known as venous insufficiency, which can cause symptoms that impact quality of life, and over time can lead to more serious problems," explained Kathleen Gibson, M.D., Lake Washington Vascular, Seattle, who performed the first treatment of a U.S. patient on October 21, 2015 with the VenaSeal closure system since approval. "Left untreated the venous insufficiency often progresses, and can cause leg pain, leg and ankle swelling, leg heaviness and fatigue, skin changes or rashes, ulcers and open wounds."
Venous insufficiency occurs when valves in the veins of the leg no longer function properly. This disease allows blood to flow backward, or reflux, resulting in enlarged, or varicose veins that become painful and can limit quality of life. Venous insufficiency affects more than 30 million Americans and is common in women who have had two or more pregnancies.
"The first patient is an active, tennis-playing mother of three with a family history of varicose veins. After careful diagnosis and evaluation of various treatment options, she and I decided VenaSeal was the right choice of treatment for her. She reported no pain during the procedure and was able to return to normal activities quickly after the treatment. She left the office with a single adhesive bandage at the site of treatment, and without post-procedure compression stockings*," said Dr. Gibson.
Using ultrasound, the physician guides a catheter through a small access site in the leg and into the diseased area of the vein. Once in place, the physician administers the VenaSeal(TM) adhesive at various points in a segmental fashion, and with manual compression, closes the vein. Blood is re-routed through other healthy veins in the leg.
This unique approach eliminates the risk of burning or nerve injury that is sometimes associated with thermal-based procedures. The procedure is administered without the use of tumescent anesthesia, minimizing the need for multiple needle sticks. In the VeClose trial, patients reported minimal - to - no pain or bruising, post procedure.
"The VenaSeal procedure is shown to be safe and effective, with consistent results across three clinical trials," said Dr. Nick Morrison, national principal investigator of the VeClose Trial, Morrison Vein Institute, Scottsdale, Ariz. "One-year results of the VeClose pivotal study, that led to the approval of VenaSeal closoure system in the U.S., continue to demonstrate safety and efficacy of the procedure, with closure rates of 97.2 percent."
"Medtronic today furthers our commitment to providing treatment options for patients with symptomatic venous reflux, a disease that can significantly impact quality of life," said Sandra Lesenfants, vice president and general manager of the endoVenous business in Medtronic's Aortic and Peripheral Vascular division. "Thousands of patients have benefited from this procedure around the world, and we are pleased to now offer this advanced technology as an option to our U.S. physicians and patients."
The VenaSeal system is currently available in the U.S., New Zealand, Chile, South Africa, Australia, Canada, Europe, United Arab Emirates and Hong Kong. To learn more visit: www.medtronic.com/endovenous
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.